Overview of Equilibrium Dialysis
Equilibrium dialysis is a specific application of dialysis that is important for the study of the binding of small molecules and ions by proteins. It is one of several methods available for this purpose, and its attractive feature continues to be its physical simplicity. Another attractive feature of equilibrium dialysis is the ability to perform interaction studies without theuse of fluorescent or radiolabeled tags.
Generally, the objective of an equilibrium dialysis experiment is to measure the amount of a ligand bound to a macromolecule. This is typically done through an indirect process because in any mixture of the ligand and macromolecule, it is difficult to distinguish between the bound and free ligand. If, however, the free ligand can be dialyzed through a membrane, until its concentration across the membrane is at equilibrium, the free ligand concentration can be measured easily. Data obtained under different experimental conditions then provides information on various binding parameters of the compounds such as the binding constants and the number of binding sites or binding capacity. 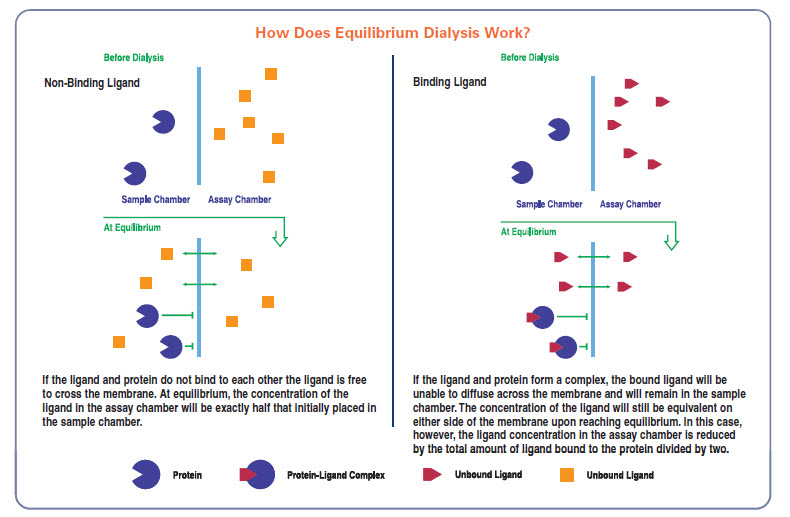
Applications
- Protein-drug binding assays
- Receptor binding assays
- Ligand binding assays
- Protein-protein interactions
- Protein-DNA interactions
- Serum protein binding
