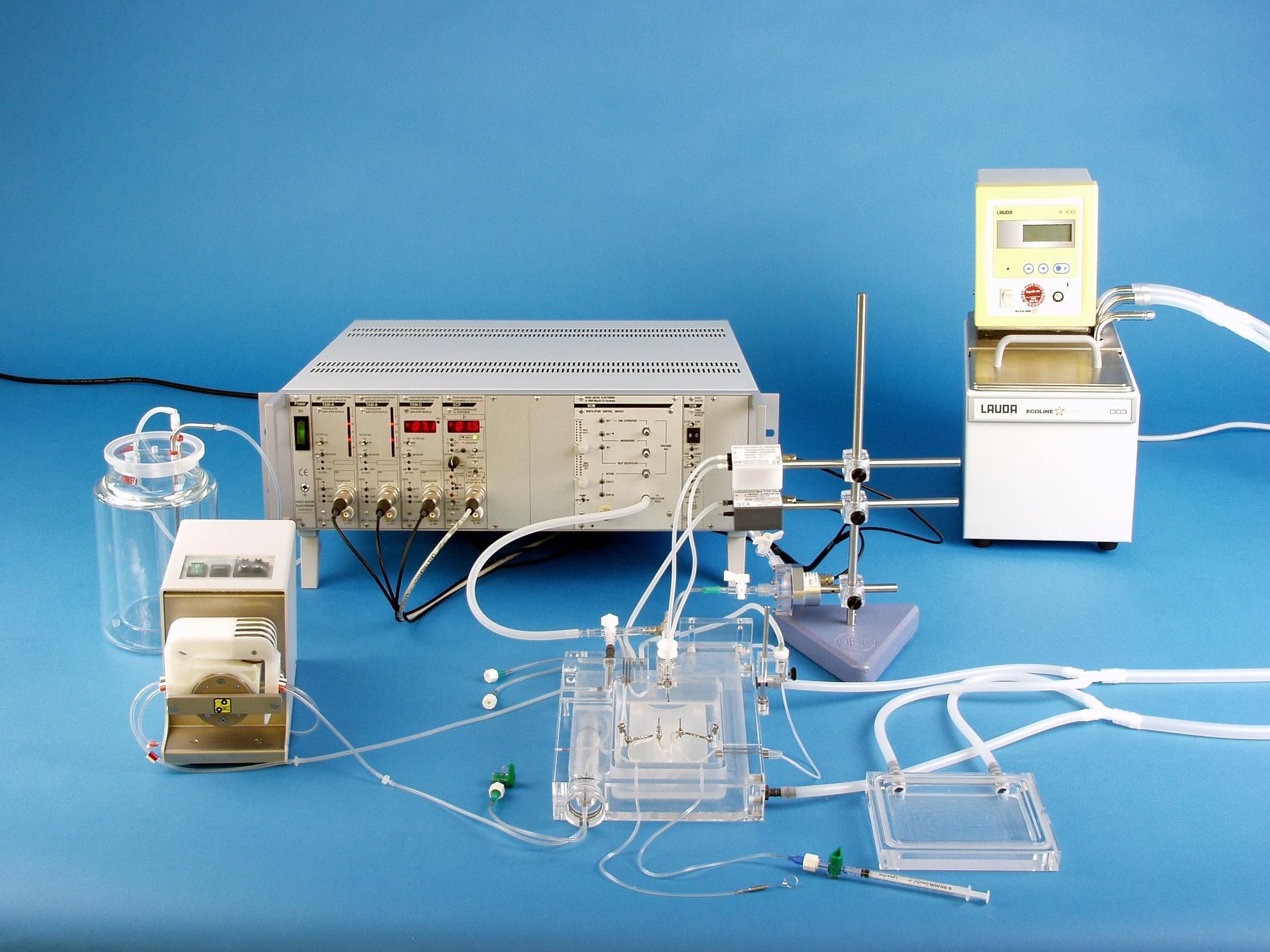
IPL-1 Core Isolated Perfused Lung System for Mouse
The IPL-1 is an ex vivo lung perfusion system (EVLP) specifically engineered for the fragile mouse lung. The core system can easily be initially configured with various system options of upgraded later are your needs change.
The core system contains all the primary equipment to accomplish basic perfusion and ventilation experiments, requiring only the addition of core options for ventilation. Application-specific upgrades and options are also available.
Due to the fragile nature of the mouse lung, its suspension from the tracheal and vascular cannulae is difficult to accomplish without the rapid occurrence of edema. In collaboration with experts in pulmonary physiology, a design was developed which allows for ventilation and perfusion with the lungs remaining in situ at a slight incline in the open thorax. This novel approach simplifies cannulation of the pulmonary artery and left atrium of the heart and dramatically reduces edema formation.
The isolated mouse lung can be ventilated under positive pressure and under subatmospheric pressure to mimic the in vivo situation as closely as possible. There are bigger differences in vascular flows seen between positive and negative ventilation. Depending on the study, a ventilated lung or a physiologic breathing lung can be simulated.
Please note: For a functional unit, the core system requires the addition of core system options for ventilation.
To ensure that your system is properly configured as a complete setup that meets your experimental needs, please email us at sales@harvardapparatus.com or call us at 800-597-0580. In Europe, please call +49 7665 92000 or email sales@hugo-sachs.de.
The IPL-1 is an ex vivo lung perfusion (EVLP) system specifically engineered for the fragile mouse lung. The core system can be initially configured with various system options or upgraded later as you needs change.
The IPL-1 core system contains all the primary equipment to accomplish the basic perfusion and ventilation experiments, requiring only the addition of core options for ventilation. Application-specific upgrades and options are also available.
Advanced System Design
Features & Benefits
Applications
Measured Signals & Calculated Parameters
Included items
Additional Requirements for a Functional Unit
Additional Components
Specialized Applications & Options
Advanced System Design
Due to the fragile nature of the mouse lung, its suspension from the tracheal and vascular cannulae is difficult to accomplish without the rapid occurrence of edema. In collaboration with experts in pulmonary physiology, a design was developed which allows for ventilation and perfusion with the lungs remaining in situ at a slight incline in the open thorax. This novel approach simplifies cannulation of the pulmonary artery and left atrium of the heart and dramatically reduces edema formation.
Most of the studies with isolated perfused lungs have been performed with lungs obtained from rats, guinea pigs, rabbits or dogs. The interaction of the immune system with the lung is currently an area of great interest. The mouse is the best characterized rodent species with respect to its immune system. Immunologic probes such as antibodies and cytokines are more widely available for mice versus other species. Likewise, the mouse is the most commonly used animal model in the biomedical research community due to the wealth of genetically modified variants for the species.
Ventilation
The HSE IPL systems for EVLP are the only commercially available systems that allow the investigator to select either positive or negative pressure ventilation. Positive pressure ventilation can be used in studies that seek to mimic clinical EVLP systems. Sub-atmospheric pressure ventilation, due to the substantial difference in vascular flow rates, allows one to more precisely mimic the in vivo physiological conditions. The lung is placed in the chamber and ventilated by either positive airway pressure or negative artificial thoracic pressure. It is connected through the tracheal cannula to the pneumotachometer for measurement of respiratory air flow. Using either positive or negative pressure, the respiration rate, the inspiratory pressure and the end-expiratory pressure can be adjusted with separate controls using the Ventilation Control Module (VCM). Positive only ventilation using a regular MiniVent ventilator is also possible. The surgery is always performed under positive ventilation to avoid the lung collapsing. Switching over to subatmospheric ventilation only requires you to attach the chamber lid and switch over a stopcock. Switching back to positive ventilation is always possible.
Perfusion
The pulmonary artery is cannulated to provide perfusion. The perfusate is passed by means of a roller pump at constant flow or constant pressure (using the SCP module) through the heat exchanger, through a bubble trap to the pulmonary artery and finally into the lung vascular bed. The perfusate outflow is usually provided by cannulating the left atrium of the heart. The non-functional lung remains in the thoracic cavity and allows easier cannula placement for the effluent.
Features & Benefits
- Exclusive artificial thorax chamber for isolated lung with integrated changeover system—quick switch between simple positive-pressure ventilation and physiological subatmospheric (negative) pressure ventilation
- Integrated surgery table—reduces damage during preparation
- Pressure-balancing vessel—eliminates transmural pressure difference during sub-atmospheric ventilation; allows for simulation of hypertensive cardiac afterload
- Built-in, warmed pneumotachometer—minimal dead space volume
- Low flow resistance and dead space volume—minimize perfusion artifacts
- Milled infusion and pressure measurement paths—allow access to closed chamber
- Built-in humidifier frit—prevents lung drying
- Moist lung chamber has access ports for additional measurements or other sensors
- Simple add-on aerosol nebulizer
- Optional multi-gas inlet adapter for hyperoxic, hypercapnic, anesthetic or other alternative gas studies
- Drug injection pathway built directly into pulmonary perfusate stream
- Cannulae are matched to size of mouse vessel and feature tip occlusion protectors and an articulated mini ball system which allow cannulae to be precisely positioned—further reducing incidence of vascular occlusion
Applications
- Investigation of ventilation and perfusion in the isolated mouse lung
- Continuous measurement of respiratory mechanics (respiration rate, inspiratory and expiratory air flow, tidal volume, minute volume, dynamic airway resistance, dynamic lung compliance) and perfusion characteristics (pulmonary artery pressure, left atrium pressure, lung vascular resistance, pO2, pCO2, pH)
- Drug testing on respiration and vascular parameters
- Toxicology tests on respiration and vascular parameters
- Aerosol tests, surfactant studies, etc.
Measured Signals and Calculated Parameters
- Pulmonary artery (perfusion) pressure
- Respiratory airflow
- Intrapleural (artificial thorax) pressure or tracheal pressure
- Perfusion flow (calculated by the SCP from pump speed or measured directly using an integrated transit time flow probe)
The following parameters can be calculated from the raw data*:
- Respiration rate
- Peak inspiratory and expiratory airflow
- Tidal volume, minute volume
- Vascular resistance
- End-inspiratory and end-expiratory pressures
- Dynamic airway resistance and dynamic lung compliance
- Inspiratory time and expiratory time (Software Option)
* Calculations are automatic when PULMODYN data acquisition software is used.
Included Items
Included items are representative of a typical IPL-1 Core System. Individual components can be customized to your needs.
| IPL-1 Core System, 230 V (73-4291) includes: | IPL-1 Core System, 115 V (73-4292) includes: | ||
| Item # | Product Name | Item # | Product Name |
| 73-2329 | Base Unit for the Mouse Isolated Perfused Lung* | 73-2329 | Base Unit for the Mouse Isolated Perfused Lung* |
| 73-4544 | TC120 Thermocirculator, complete with 5 L stainless steel bath and lid, 220 V | 73-4545 | TC120 Thermocirculator, complete with 5 L stainless steel bath and lid, 120 V |
| 73-3436 | Water-Jacketed Glass Perfusate Reservoir with Oxygenating Frit, 0.5 L | 73-3436 | Water-Jacketed Glass Perfusate Reservoir with Oxygenating Frit, 0.5 L |
| 73-3456 | Tube Set for Jacketed Buffer Reservoir with Fluid Line Shutoff Valves | 73-3456 | Tube Set for Jacketed Buffer Reservoir with Fluid Line Shutoff Valves |
| - | Reglo Peristaltic (Roller) Pump | - | Reglo Peristaltic (Roller) Pump |
| 73-1825 | 3-Stop Tygon® E-Lab Tubing, 0.89 mm ID, 12/pack, Orange/Orange | 73-1825 | 3-Stop Tygon® E-Lab Tubing, 0.89 mm ID, 12/pack, Orange/Orange |
| 73-0126 | 3-Stop Tygon® E-Lab Tubing, 1.22 mm ID, 12/pack, Red/Grey | 73-0126 | 3-Stop Tygon® E-Lab Tubing, 1.22 mm ID, 12/pack, Red/Grey |
| 73-0500 | Lab Stand with Triangular Base Plate, 30 cm Rod Length (one block clamp included) | 73-0500 | Lab Stand with Triangular Base Plate, 30 cm Rod Length (one block clamp included) |
| 73-0566 | Block Clamp to Mount Second Transducer onto 73-0500 Stand (up to 9 mm OD) | 73-0566 | Block Clamp to Mount Second Transducer onto 73-0500 Stand (up to 9 mm OD) |
| 73-0045 | PLUGSYS Case, Type 603 | 73-0045 | PLUGSYS Case, Type 603 |
| 73-2806 | Servo Controller for Perfusion (SCP) | 73-2806 | Servo Controller for Perfusion (SCP) |
| Perfusion Pressure Measurements | |||
| 73-0020 | Low Range Blood Pressure Transducer P75 for PLUGSYS Module | 73-0020 | Low Range Blood Pressure Transducer P75 for PLUGSYS Module |
| 73-1793 | PLUGSYS Transducer Amplifier Module (TAM-D) | 73-1793 | PLUGSYS Transducer Amplifier Module (TAM-D) |
| Respiratory Airflow Measurements | |||
| 73-3882 |
Differential Low Pressure Transducer DLP2.5, Range ± 2.5 cmH2O, HSE Connector | 73-3882 | Differential Low Pressure Transducer DLP2.5, Range ± 2.5 cmH2O, HSE Connector |
| 73-0065 | PLUGSYS Transducer Amplifier Module (TAM-A) | 73-0065 | PLUGSYS Transducer Amplifier Module (TAM-A) |
| Artificial Thorax or Tracheal Pressure Measurements | |||
| 73-0064 | Airway/Thoracic Pressure Transducer MPX, Range ± 100 cmH2O, HSE Connector |
73-0064 | Airway/Thoracic Pressure Transducer MPX, Range ± 100 cmH2O, HSE Connector |
| 73-0065 | PLUGSYS Transducer Amplifier Module (TAM-A) | 73-0065 | PLUGSYS Transducer Amplifier Module (TAM-A) |
The Base Unit (73-2329) includes:
Temperature-controlled negative pressure lung chamber (artificial thorax) with cover including Venturi jet, positive pressure ventilation head, air humidifier, pneumotachometer, connectors to interface with the perfusionsystem, pressure equilibration vessel for venous flow and all necessary accessories (tracheal cannulae, pulmonary artery cannulae, left atrium cannulae). Optional Y adapter available for positive pressure ventilation.
Mouse Cannulae (included with Base Unit):
- Tracheal Cannula for Mouse, ID 1.0 mm, OD 1.3 mm, L 20 mm (73-4181)
- Pulmonary Artery Cannula for Mouse, Stainless Steel, ID 1.0 mm, OD 1.3/1.6 mm, L 28 mm (73-0723)
- Atrial Cannula for Mouse, ID 1.0 mm, OD 1.6 mm, L 24 mm (73-0724)
Additional Requirements for a Functional Unit
For a functional IPL-1 unit the core system requires a suitable ventilation system. For only positive ventilation a mouse ventilator can be used. If positive and sub-atmospheric pressure ventilation is required, the Ventilation Control Module must be purchased.
Standard Options for the Core IPL-1 System (Purchase Separately)
PULMODYN Data Acquisition Software
Online evaluation of a wide range of signals and classical respiration parameters.
Note: Ponemah Data Acquisition & Analysis Software from DSI, a Harvard Bioscience Company is also suitable.
Specialized Applications & Options
Venous pressure transducer and amplifier for venous pressure measurement in an isolated perfused lung system.
For deoxygenation of blood or buffers containing proteins (e.g. albumin) or erythrocytes.
Delivers CO2 to maintain pH when system is not deoxygenated with N2/CO2 gas mixture.
Permits precise continuous or discontinuous measurement in liquid media or perfusate of these three key parameters: pO2, pH and pCO2 .
Measure perfusate temperature in any isolated perfused organ system.
For filtration of recirculated perfusate.
Add-on to deliver aerosol to the isolated lung.
For accurate drug addition using a syringe pump. Additional option for flow controlled drug addition, where flow is measured (or calculated) and a drug must be added in a certain ratio.
IPL-1 References
Mehaffey J, et al. (2018). Increasing circulating sphingosine-1-phosphateattenuates lung injury during ex vivo lung perfusion. J Thorac Cardiovasc Surg.156 (2):910-917.
Stone ML, et al. (2017). Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res. 18 (1):212.
Huerter ME, Sharma AK, Zhao Y, Charles EJ, Kron IL & Laubach VE. (2016). Attenuation of Pulmonary Ischemia-Reperfusion Injury by Adenosine A2B Receptor Antagonism. Ann Thorac Surg. 102(2):385-393.
Jahn N, et al. (2015). Inhaled carbon monoxide protects time-dependently from loss of hypoxic pulmonary vasoconstriction in endotoxemic mice. Resp Res. 16(1):119.
Patel BV, Tatham, KC, Wilson MR, O'Dea KP & Takata, M. (2015). In vivo compartmental analysis of leukocytes in mouse lungs. Amer J Physiol Lung Cell Mol Physiol. 309 (7):L639-L652.
Stone ML, et al. (2015). Ex Vivo Perfusion with Adenosine A2A Receptor Agonist Enhances Rehabilitation of Murine Donor Lungs after Circulatory Death. Transplantation. 99 (12):2494-2503.
Fernandez LG, Sharma AK, LaPar DJ, Kron IL & Laubach VE. (2013). Adenosine A1 receptor activation attenuates lung ischemia–reperfusion injury. J ThoracCardiovasc Surg. 145 (6):1654-1659.
Yin J, et al. (2013). Vasodilatory Effect of the Stable Vasoactive Intestinal Peptide Analog RO 25-1553 in Murine and Rat Lungs. PLoS ONE. 8 (9):E75861.
Yoo HY, et al. (2013). Optimization of Isolated Perfused/Ventilated Mouse Lung to Study Hypoxic Pulmonary Vasoconstriction. Pulm Circ. 3 (2):396-405.
Dong M, et al. (2012). Pulmonary delivery and tissue distribution of aerosolized antisense 2'-O-Methyl RNA containing nanoplexes in the isolated perfused and ventilated rat lung. Eur J Pharm Biopharm. 81 (3):478-485.
Engelberts D, Malhotra A, Butler JP, Topulos GP, Loring SH & Kavanagh BP.(2012). Relative effects of negative versus positive pressure ventilation depend on applied conditions. Intensive Care Med. 38 (5):879-885.
Vanderpool RR , El-Bizri N, Rabinovitch M & Chesler NC. (2012). Patchydeletion of Bmpr1a potentiates proximal pulmonary artery remodeling in miceexposed to chronic hypoxia. Biomech Model Mechanobiol. 12 (1):33-42.
Weissmann, N, et. al. (2012). Activation of TRPC6 channels is essential for lung ischaemia–reperfusion induced oedema in mice. Nat Comm. 3:649.
Keseru B, et al. (2010). Hypoxia-induced pulmonary hypertension: comparison of soluble epoxide hydrolase deletion vs. inhibition. Cardiovasc Res.85 (1):232-240.
Seimetz M, et al, (2011). Inducible NOS Inhibition Reverses Tobacco-Smoke-Induced Emphysema and Pulmonary Hypertension in Mice. Cell. 147:293-305.
Vanderpool RR & Chesler NC. (2011) Characterization of the isolated,ventilated and instrumented mouse lung perfused with pulsatile flow. J VisExp. Apr 28; (50): pii: 2690. doi: 10.3791/2690.
Vanderpool RR , Kim AR, Molthen R & Chesler NC. (2011) Effects of acute rhokinase inhibition on chronic hypoxia-induced changes in proximal and distal pulmonary arterial structure and function. J App Physiol. 110 (1):188-98.
Busch C, et al. (2010). Effects of ketamine on hypoxic pulmonary vasoconstriction in the isolated perfused lungs of endotoxaemic mice. Eur JAnaesthesiol. 27 (1):61-66.
Vanderpool R, Naeije R & Chesler N. (2010). Impedance in isolated mouse lungs for the determination of site of action of vasoactive agents and chronichypoxia. Ann Biomed Eng. May;38 (5):1854-1861.
Spohr F, Busch C, Teschendorf P & Weimann J. (2009). Selective inhibition ofguanylate cyclase prevents impairment of hypoxic pulmonary vasoconstriction in endotoxemic mice. J Physiol Pharmacol. 60 (2):107-112.
Tang B, et al. (2009). Endothelin-1 inhibits background two-pore domain channel TASK-1 in primary human pulmonary artery smooth muscle cells.Amer J Respir Cell Mol Biol. 41 (4):476-483.
Vanderpool R, Naeije R & Chesler N. (2009). Impedance in isolated mouse lungs for the determination of site of action of vasoactive agents and disease.Ann Biomed Eng. 38 (5):1854-1861.
Weissmann N, et al. (2009). The soluble guanylate cyclase activator HMR1766 reverses hypoxia-induced experimental pulmonary hypertension in mice. Am JPhysiol Lung Cell Mol Physiol. 297 (4): L658-L665.
Spohr F, et al. (2007). 4-Aminopyridine Restores Impaired Hypoxic Pulmonary Vasoconstriction in Endotoxemic Mice. Anesthesiol. 107 (4):597-604.
Tuchscherer HA, Vanderpool RR & Chesler NC. (2007). Pulmonary vascular remodeling in isolated mouse lungs: Effects on pulsatile pressure–flow relationships.
J Biomechan. 40 (5):993-1001.
Dolinay T, et al. (2006). Gene expression profiling of target genes in ventilator inducedlung injury. Physiol Genomics. 26 (1):68-75.
Spohr F, et al. (2005). Role of endogenous nitric oxide in endotoxin induced alteration of hypoxic pulmonary vasoconstriction in mice. Am J Physiol HeartCirc Physiol. 289 (2):H823-H831.
Hasegawa J, et al. (2004). Altered Pulmonary Vascular Reactivity in Mice with Excessive Erythrocytosis. Am J Respir Crit Care Med. 169 (7):829-835.
Maxey T, et al. (2004). Tumor necrosis factor-a from resident lung cells is a key initiating factor in pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 127 (2): 541-547.
Tuchscherer H, Webster E & Chesler N. (2004). Pulmonary vascular resistance and impedance in isolated mouse lungs: Effects of pulmonary emboli. Ann Biomed Eng. 34 (4):660-68.
Uhlig U, et al. (2004). Phosphoinositide 3-OH kinase inhibition prevents ventilation-induced lung cell activation. Am J Respir Crit Care Med.169 (2):201-208.
Weissmann N, et al. (2004). Basic features of hypoxic pulmonary vasoconstriction in mice. Respir Physiol Neurobiol. 139 (2):191-202.
Kuebler W, et al. (2003). Stretch activates nitric oxide production in pulmonary vascular endothelial cells in situ. Am J Respir Crit Care Med. 168 (11):1391-1398.
Stamme C, Brasch F, von Bethmann A & Uhlig S. (2002). Effect of surfactant on ventilation-induced mediator release in isolated perfused mouse lungs. Pulm Pharmacol Ther. 15 (5):455-461.
Held H, Boettcher S, Hamann L & Uhlig S. (2001). Ventilation-Induced Chemokine and Cytokine Release Is Associated with Activation of Nuclear Factor-kappaB and Is Blocked by Steroids. Am J Respir Crit Care Med. 163(3):711-716.
Held H & Uhlig S. (2000). Basal lung mechanics and airway and pulmonary vascular responsiveness in different inbred mouse strains. J App Physiol. 88(6):2192-2198.
Held H & Uhlig S. (2000). Mechanisms of endotoxin-induced airway and pulmonary vascular hyperreactivity in mice. Am J Respir Crit Care Med. 162(4):1547-1552.
von Bethmann A, et al. (1998). Hyperventilation induces release of cytokines from perfused mouse lung. Am J Respir Crit Care Med. 157 (1):263-272.