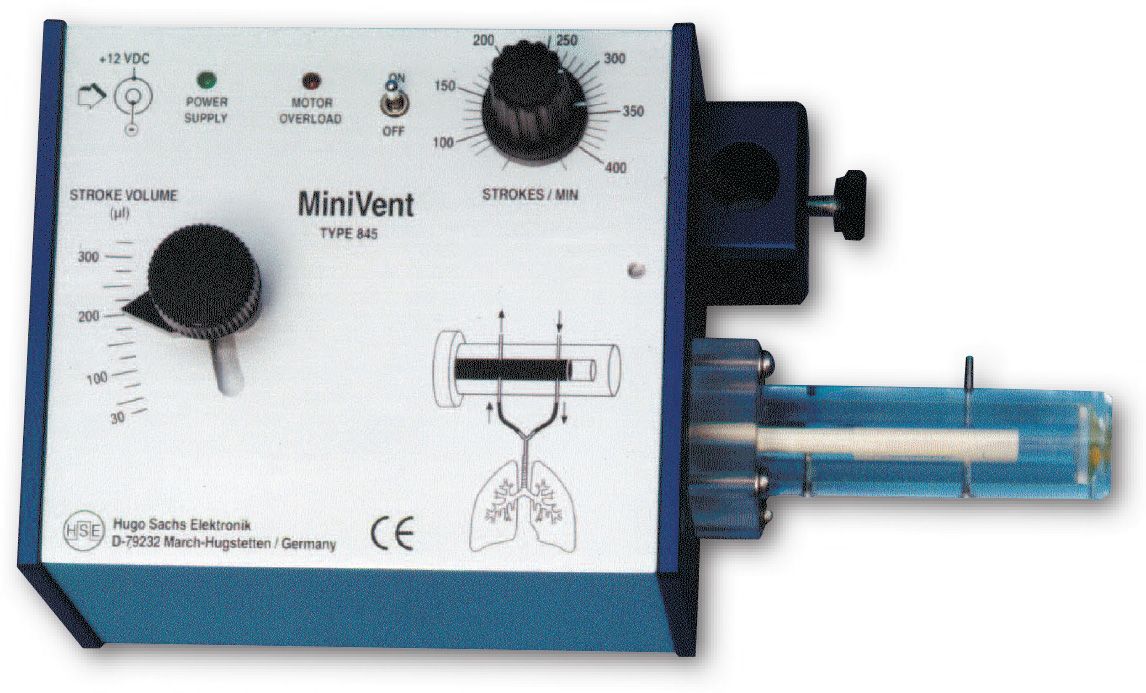
MiniVent Ventilator for Mice (Model 845), Single Animal, Volume Controlled
The MiniVent Model 845 Ventilator is a quiet, compact and light weight ventilator. While it was designed specifically for mice, the MiniVent can be used for any animal (e.g. birds and perinatal rats) which requires tidal volumes in the range of 30 to 350 µl and respiratory rates of 60 to 400 breaths per minute.
- Ideal ventilator for mice
- Stroke volume range from 30 to 350 µl
- Ventilation rate from 60 to 400 breaths/minute
- Simple adjustment of stroke volume while running
- Valveless piston pump, no valves to clog
- Very small instrument/circuit dead space volumne
- Compact construction, easy to install close to animal
- No vibrations, very low noise
Please see the item listing for accessories and replacement parts.
The MiniVent Model 845 Ventilator is a quiet, compact and light weight ventilator designed specifically for mice. It can be used for any animal (e.g. birds and perinatal rats) which requires tidal volumes in the range of 30 to 350 µl and respiratory rates of 60 to 400 breaths per minute.
Key Features
- Ideal ventilator for mice
- Stroke volume range from 30 to 350 µl
- Ventilation rate from 60 to 400 breaths/minute
- Simple adjustment of stroke volume while running
- Valveless piston pump, no valves to clog
- Very small instrument/circuit dead space volumne
- Compact construction, easy to install close to animal
- No vibrations, very low noise
How it Works
The MiniVent Ventilator is a constant-volume respiration pump operating on the Starling principle. Unlike conventional units for larger animals, this ventilator employs a rotary plunger and has no valves. During each ventilation cycle, the plunger performs a synchronized forward and rotating movement. Cleverly arranged bores and channels in the cylinder and plunger control inspiration and expiration during each stroke of the plunger.
The extremely light weight and compact construction, in addition to the convenient rod clamp, allow the MiniVent ventilator to be positioned directly next to the animal. Typical setups with larger ventilators produce large tubing and instrument dead space volumes. These larger volumes introduce greater system compliance which can affect the accuracy with which the full tidal volume is introduced into the animal’s lungs. With the MiniVent, the tidal volume error due to system compliance is reduced to ±3 µl.
Tidal volume and respiration rate can be set exactly to the values required for mouse ventilation. The level of precision and control available to the investigator minimizes the danger of hyperventilation or hypoventilation.
The tidal volume can be varied continuously from 30 to 350 µl during operation without having to interrupt ventilation. The respiration rate is also continuously adjustable from 60 to 400 strokes/min. The expired air can be recovered at the collection port for sampling, recycling or for the generation of a positive end-expiratory pressure (PEEP). Room air or any non-explosive gas mixture can be used to feed the pump intake.
Components
The MiniVent is supplied with 1 x AC Wall Mounted Power Supply (115 V or 220 V); 2 x Silicone Tubing (1.5mm ID, 3.0mm OD, 14cm long); 1 x 1.3mm OD Tracheotomy Cannula (73-2730); 1 x 1.2mm OD Intubation Cannula (73-2844).
A multi-gas inlet adapter is available for the MiniVent so that alternate gas mixtures and nebulized substances are delivered to the MiniVent inlet port at atmospheric pressure. The adapter provides ports for multiple selectable gas mixtures (hypoxic, anesthetic...) and a port for the Aerosol Nebulizer.
| Voltage Range 73-0043 73-0044 |
115 VAC 230 VAC |
| Number of Animals | 1 |
| Species | Mouse, prenatal rat, small bird |
| Control Modes | Volume |
| Gas Supply | Room air or non-flammable mixed gas |
| Display | None |
| IE Ratio,% | 1:1 |
| PEEP | Provided via attachment of water column |
| Respiration Rate | 60 (min) to 400 (max) breaths/min |
| Respiration Rate Note | Continuously adjustable from 60 to 400 breaths/min |
| Sigh Frequency | None |
| Sigh Pressure | Not available |
| Tidal Volume | 0.03 (min) to 0.13 (max) ml/stroke |
| Stroke Volume | Continuously adjustable from 30 to 350 µl |
| Weight Range | 1 (min) to 50 (max) g |
| Dimensions, W x H x D) | 3.9 x 3.1 x 7.9 in (10 x 8 x 20 cm) |
| Net Weight | 2.2 lb (1 kg) |
| AC Adapter Weight | 0.7 lb (0.3 kg) |
| Certifications | CE |
Cannulae
| 73-2731 | 73-2730 | 73-2844 | 73-4112 | |
| Animal Species | Mouse | Mouse | Mouse | Small Rat |
| Length, mm | 13 | 13 | 30 | 15 |
| Note | Length listed is total cannula length | Length listed is total cannula length |
Length listed is total cannula length |
With the medium Y-adapter (7.5 mm OD), this cannula length is 3 mm less than listed |
| OD, mm | 1.0 | 1.3 | 1.2 | 1.5 |
| Y-Adapter, mm | 3.0 | 3.0 | 3.0 | 7.5 |